
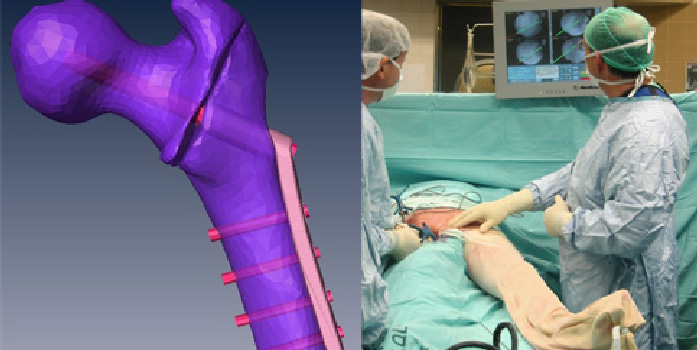
- Offer Profile
- Our main focus is on computer-aided orthopaedic surgery and related applications. Specifically, we are developing techniques for model construction and visualization of volumetric medical data, preoperative planning and visualization, fluoroscopic X-ray and ultrasound image processing, anatomy-based registration, and image-guided and robot-based navigation.
New Projects: Interventional endovascular procedures simulation with patient-specific carotid arteries models generated from CTA scans
- 1. School of Eng. and Computer Science, The Hebrew
Univ. of Jerusalem, Israel.
2. Simbionix LTD, Israel.
3. Dept of Radiology, School of Medicine, Hadassah Hebrew U, Medical Center, Israel.Purpose
Minimally invasive endovascular surgeries such as carotid, coronary, and cardiac angiographic procedures are frequent in interventional radiology. They require experienced physicians and involve time-consuming trial and error with repeated contrast agent injection and X-ray imaging. This leads to outcome variability and non-negligible complication rates. Training simulators such the ANGIO MentorTM (Simbionix LTD. Israel, 2008) have the potential to significantly reduce the physicians’ learning curve, improve their performance, and reduce the outcome variability. A key limitation is the simulators’ reliance on hand-tailored anatomical models generated by a technician from CTA scans, which are impractical to produce for each patient in a clinical environment.Patient specific simulation requires the segmentation of the entire vascular anatomy including the Common Carotid Artery (CCA), the extracranial Internal Carotid Artery (ICA) and External Carotid Artery (ECA), the ECA branches, the Carotid Bifurcation (CB), the Subclavian Arteries (SA), and the Aortic Arch (AA). The vertebral arteries are desired but not required for the simulation. These vessel structures usually have a very large intra and inter-patient intensity and geometrical shape variability, are near bone structures with similar intensity values, and suffer from imaging artifacts caused by metallic objects such as dental implants. In addition, in many pathological cases, severe stenosis around the carotid bifurcation frequently causes segmentation failure. Therefore the generation of patient specific models for simulation is a challenging task.
Materials and Method
In this study, we conducted a preliminary evaluation of the resulting segmentation models using our new method for patient-specific carotid interventional radiology simulations. The method starts with the morphological-based segmentation of the aorta and the construction of a prior intensity probability distribution function for arteries. The carotid arteries are then segmented with a graph min-cut method based on a new edge weights function that adaptively couples voxel intensity, intensity prior, and geometric vesselness shape prior. Finally, the same graph-cut optimization framework is used to interactively remove a few vessel segments and to fill minor vessel discontinuities caused by intensity variations.
In the study, we used a Simbionix ANGIO MentorTM (Simbionix LTD. Israel, 2008) station. The ANGIO MentorTM is an integrated software and hardware endovascular simulation platform (Fig. 1a). It simulates interventional vascular procedures based on a diagnostic CTA and a vasculature simulation model. It supports realistic haptic catheter insertion and manipulation feedback (Fig. 1b) and creates continuous fluoroscopic X-ray imaging, fluoroscopic C-arm positioning, and simulated contrast agent injection (Fig. 2a-c). For more details, see http://www.simbionix.com
Four CTA datasets acquired with administrated 100cc of non-iodinated of contrast agent with a rapid injection aid at 3-4cc per sec. The CTA scans, acquired on a Sensation 16 Siemens Medical Solutions scanner (Forchheim, Germany) have in-plane pixel size 0.5x0.5mm2, matrix size 512x512, 0.55mm slice spacing, and 750 slices. The patient specific models were generated from the CTA images as follows. First, the carotid arteries systems were segmented using a nearly-automatic method, 3D mesh with centerlines, bifurcation points, and vascular radiuses were computed with the VMTK software library automatic meshing and centerline generation modules. The entire simulation model generation required less than 10 minutes for each on a standard PC, most of it computation time without interaction.Results
The simulation models were then directly transferred to the Simbionix ANGIO MentorTM simulator platform. We then performed common interventional radiology procedures, such as catheter insertion and manipulation, balloon positioning and dilation, and stent placement on the patient-specific models. Fig. 2a-c shows sample snapshots of the simulation with the patient-specific models.
A movie showing the simulation with our 3D models is available in http://www.cs.huji.ac.il/~freiman/vessels-cut
The simulations ran flawlessly and successfully in real time for over an hour. The users reported great realism and an excellent overall experience, which was significantly better than similar experiences with the previous manually generated models. While this simulation experiment is qualitative and preliminary, it constitutes a proof-of-concept of practical patient specific carotid interventional radiology simulations from clinical CTA scans.
Conclusion
We have presented a proof-of-concept of practical patient specific carotid interventional radiology simulations from clinical CTA scans. Patient specific models were generated using previously presented techniques, and used for clinical simulation successfully. Our results indicate that the generated models are accurate, robust, and provide useful information for patient specific simulation.
We are currently expanding the using of patient specific models for intra-operative mode guided interventional radiology procedures. 
Fig. 1a: Simbionix ANGIO MentorTM

Fig. 1b: Haptic catheter manipulation.
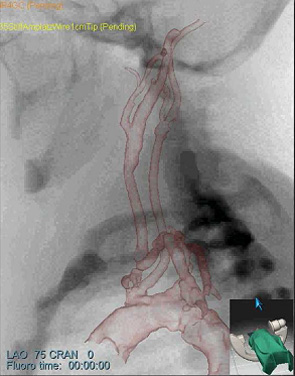
Fig. 2: Patient-specific simulation experiment
- (a) Lateral simulated angiogram
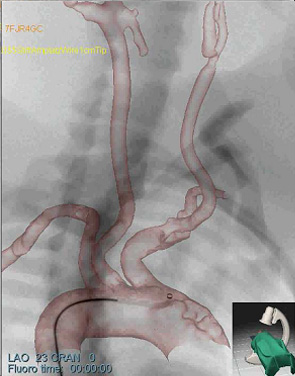
Fig. 2: Patient-specific simulation experiment
- (b) AP simulated angiogram
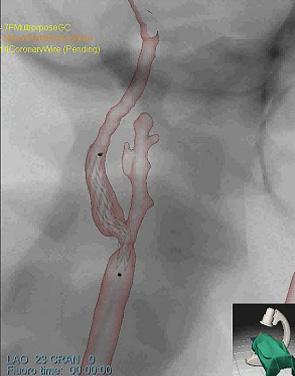
Fig. 2: Patient-specific simulation experiment
- (c) bifurcation with stent – detail
Research Projects
Orthopaedics
-
1.Navigated metal detector probe for shrapnel removal
2.Patient-specific fracture CAD/CAM
3.Pelvic fracture reduction and fixation with EM tracking
4.Model-based reconstruction from a few X-rays
5.Evidence-based Computer-Aided Orthopaedic Surgery
Radiology
-
1.Liver tumor and vessels visualization
2.Early cancer detection in MRI images
3.Registration of multiple sclerosis MRIs
4.Patient-specific preoperative simulation of endovascular surgical procedures
Neurosurgery
-
1.Miniature robot system for keyhole neurosurgery
2.Surface and landmark-based registration for IGS
3.Augmented reality probe for intraoperative navigation
Generic technologies
-
1.Quantification of geometric uncertainty
2.Nearly-automatic segmentation of vascular anatomical structures
3.Virtual and augmented reality applications in surgery
4.Multimodal rigid and non-rigid registration algorithms
Active grants
-
- ROBOCAST: Robot and sensor integration as guidance for enhanced computer-assisted surgery and therapy. 7th European Union Program, Consortium of 8 univesities and 2 companies in Italy, Germany, UK, and Israel, 2007-10.
- Patient-specific preoperative simulation of endovascular surgical procedures, with Simbionix Ltd. and Dr. Sosna, Dept of Radiology, Hadassah. Ministry of Trade and Industry, MAGNETON Grant 38652, 2007-09.
- Computer-aided intraoperative fracture reduction and fixation based on electromagnetic tracking, with M. Liebergall, Dept of Orthopaedic Surgery, Hadassah, Innovation Grant, the Hebrew University, 2006-07.
-
Collaboration
Current academic and clinical Israeli collaborations:
-
Hadassah Ein Karem:
Departments of Radiology, Neurosurgery, Orthopaedics, and Institute for Gene
Therapy
Multiple Sclerosis Center, Sheba Medical Center
Laboratory for Robotics, Faculty of Mechanical Engineering, Technion
Current collaborations with Israeli industry
-
- Simbionix
- Mazor Surgical Technologies
Recent awards
Title
-
Kaye Innovation Award, The
Hebrew University of Jerusalem, June 2007.
Best Clinical Paper presentation, 6th Annual Meeting, Int. Society for Computer-Aided Orthopaedic Surg ery, June 21-24, 2006, Montreal, Canada.
Outstanding Israeli Project, European Union 7th Framework Programme for Research and Technological Development. Awarded by the European Commission to the State of Israel, October 2007