
- Offer Profile
- DE DIETRICH PROCESS
SYSTEMS
is the leading global provider of Process Equipment, Engineered Systems and Process Solutions for the fine chemical, chemical and pharmaceutical industry.
Reaction

-
For highly corrosive materials or end products that require ultra-pure environments, De Dietrich Process Systems reactors are the best choice to achieve process performance goals efficiently and economically.
We are able to provide complete engineered solutions for reaction. Our reactors are available as standalone equipment or can be incorporated into a system, including upstream and downstream equipment.
As a chemical process equipment specialist, our main purpose is to ensure both the mixture of reactants and heat transfer, resulting in a guaranteed process efficiency.
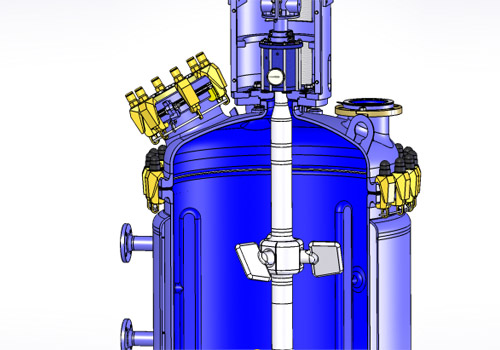
Reaction units are primarily manufactured from glass-lined steel, borosilicate 3.3 glass or stainless steel with a variety of accessories and instrumentation. Each method of construction has its own advantages for process and operation considerations. GLASS-LINED REACTOR
- Our glass-lined reactors have been at the heart of chemical operations for over a century. These are robustly designed and manufactured to stand up to very harsh environments.
THE DIN RANGE
-
This range of reactors meets every standards and is able to cover all your needs whatever your process. The perfect control of our manufacture and quality procedures leads to provide high quality products every time.
The polyvalence and reliability in every circumstances
All our glass lined-steel reactors are in accordance with the DIN 28136.
Three types of glass-lined steel reactor design AE, BE, CE which can be provided with jacket or half-coil and a wide selection of agitation solutions.
BE
closed-welded reactors without a large main opening for higher pressure ratings.
AE
clamped or flanged-top reactors designed with a removable top head for today’s cleanability demands.
CE
rugged, heavyweight reactors with large top head main cover that allows for installation and removal of one-piece agitators.

THE OPTIMIX® REACTOR
-
Exclusively from De Dietrich Process Systems, the OptiMix® reactor offers tremendous optimization of the mixing performance of glass-lined steel reactors by integrating three baffles on the vessel wall.
OptiMix® reactor: The efficient technology
This patented design enhances heat transfer, solids suspension and distribution, gas dispersion, gas flow rates and mass transfer.
The OptiMix glass-lined reactor's performances are based on the integration of three baffles onto the vessel wall. This leads to a notable improvement in the capacity of the mixture and in heat transfer compared to the standard DIN series.
The result is that all the nozzles remain free for the process. This arrangement considerably improves the hydrodynamics by using an optimized baffling effect.
The profile of the paddles prevents the accumulation of crystals in the event of crystallization. This design satisfies two of industry's primary requirements: optimal process efficiency and improved cleanability of the reactor, as well as a reduction of the amount of dead zones which generally occurs with a standard, nozzle-mounted baffle.
CHARACTERISTICS- OptiMix reactors are lined with DD3009 glass for superior corrosion resistance.
- A complete range standardized from 6.3l (glass) up to 25m3 (Glass-lined).
- Working conditions:
- Pressures from full vacuum to 6/10 bar
- Temperatures from -30°C to 260°C
- The OptiMix reactor range is fitted with an integrated sensor on the shell which enables control of very small reaction volumes.
- Equipment is interchangeable with standard reactors of the same size. OptiMix comes standard with two coil zones and a GlasLock® agitator, with optional jacket.

PHARMA REACTOR
-
Innovative reactors provide optimal cleanability
Advanced solutions for efficient cleaning
CHARACTERISTICS- A wide range from 63 l up to 630 l
- Optimization of the thermal transfer and the mixing performances (OptiMix®)
- Glass-lined flat cover with excellent surface finish and pad nozzles
- Polished 316L Stainless Steel sheathing on drive and motor
- CIP validation
- Successful test results
ADVANTAGES- OptiMix design for high agitation performances, more connections and less dead zones
- A range of advanced solutions like:
- Fused Glass quick release opening (by request)
- Flat seal outlet valve
- Pad nozzles
- Reversed mechanical seal
- High performing spray balls: different materials available (in Alloy or PTFE)

EURO EZ
-
The Euro EZ reactor has been designed for easy processes but still feature the same level of quality found in the DIN range. This guarantees equipment reliability.
The economical solution for easy processes
For this design, new calculation data is taken into account which allows us to reduce steel thickness and weight, the number of clamps, and the sizing of agitation and drive assemblies.
Standardization has been deeply applied to this range of products, which gives you at the end a reactor that competently fulfills basic process requirements, in a total safety and reliability, for lower investment costs.
CHARACTERISTICS- Pressure: internal -1 to 3 bar ; jacket 4 bar
- Temperature: -10 to 150° C
- Viscosity: max. 500 cP
- Specific weight: 1250 kg/m3
- Codes: ASME - AD2000 - CODAP
ADVANTAGES- De Dietrich DD3009 high enamel quality
- Nozzles with different connections possibilities (DIN, ANSI, JIS…)
- Specific drive with direct coupling, dry single mechanical seal, (C/SiC) and gearbox motor
- Reduced maintenance costs
- Impeller agitator and baffle equipped with DR2 temperature probe
- Possibility of support by legs or lugs
- Jacket equipped with agitation nozzles
GLASS REACTOR
-
Our range of reactors and components, manufactured from Borosilicate Glass 3.3, provide excellent resistance to chemical attack and corrosion as well as transparency for optimum visual observation.
- PERFECT OBSERVATION OF THE PROCESS -
- UNIVERSAL USE due to :
- high corrosion resistance-
- wide temperature range -
- vacuum resistance -
- EASY CLEANING due to:
- transparency -
- smooth surface - 
UNIVERSAL REACTOR
-
The Universal-Reactor is the solution for synthesizing quantities that are too large for a lab scale 3-neck flask.
The complete solution if the 3 neck flask becomes too small.
HIGHLIGHTS
- Reaction unit including EX-instrumentation
- Easy cleaning due to self-draining construction
- Certificates for material in contact with product
- Robust stirrer drive
- For operation in EX-rated areas
FUNCTION
The addition of the reactants into the reactor is done through the hand whole equipped with a quick release closure. By applying vacuum to the feed vessel another reactant can be sucked into the feed vessel and afterward dosed through a dip pipe directly into the liquid reaction phase. The valve assemblies permit to operate the complete unit as well as single receivers under vacuum or slight inert gas overpressure up to +0.5barg.

BIOREACTOR
-
A new range of reactors from 5 to 20 liters developed with users to increase cell culture yields
GMP REACTOR- Borosilicate 3.3 Glass according EN1595 with or without jacket
- Monobloc inner vessel, ≤ Ra 0.02 μm
- Customized design (Optimization of the KLa)
AGITATION- Minimization of agitation restrictions (CFD)
- Mechanical or magnetic
COVER & ACCESSORIES- Stainless steel 316 L or Alloy
- Ferrite content < 5%
- Electro polished finish Ra < 0.5 μm
- EPDM gaskets with FDA, USP certificates
SERVICES- QA / QC plan
- FAT, SAT
- Qualification : DQ, IQ, OQ
- Aftersales and spare parts
DOCUMENTATION- According to GMP requirement
CONTROL / SUPERVISION- Entry level with variable speed control
- 21 CFR part11 Control interface (on request) pH, RedOx, T°, aeration (KLa), stirring speed,...
MIXING
- Efficient mixing is essential to the reaction process and is the major concerns of the chemical and pharmaceutical industries. Reliable agitation, mixing and heat transfer solutions are necessary to improve performances and reduce production costs.

THE GLASLOCK® SYSTEM
-
The GlasLock system allows installation of specific blade profiles as well as several flights of blades to better suit the process requirements. This system, therefore, makes it possible to obtain small, non-agitated volumes less than 1%.
The only system in the world with removable blades
This system has been especially designed for BE reactors where the main opening is the manhole, but is usable for all kinds of reactors.
Fitting and dismantling are carried out laterally, making it possible to work on the scraping agitators without completely dismantling them. With multi-tiered agitators, it is possible to modify just one stage independently of the others by leaving the agitator in place.
The assembly of a blade in its conical emplacement, both fully enamelled, is done using a manual tool according to a simple procedure. GlasLock uses a single hub offering greater flexibility by allowing all types of blade profiles to be fitted. This technology reduces the time needed to dismantle the drive, when this is required.
Our teams can define and install a blade profile according to your process and your agitation parameters. This technology makes it possible to fit several flights of blades to better suit the reaction.
ADVANTAGES- Multi process-system
- Individual dismantling of the blades
- Small agitated volume
- Easy maintenance
- Limited stock of blades
MONOBLOC AGITATORS
-
This range of agitators, covered with our DD3009 enamel, is dedicated to reactors featuring a large opening (AE and CE reactors)
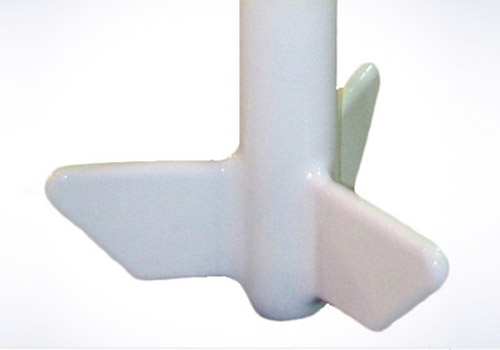
IMPELLER AGITATOR
The Impeller is an universal agitator giving a radial flow.
Having three curved blades, it works between 50 and 200 RPM.
ANCHOR AGITATOR
The Anchor is an agitator specially used in viscous products and to increase the heat transfer. Its shape is adapted to the reactor. Low rotation speeds, from 20 to 80 RPM.
TURBINE AGITATOR
The Turbine is specifically adapted for dispersion reactions. Having three to six welded blades, it can work up to 500 RPM.
FRAME AGITATOR
The Frame can be used for homogenization on a large range of viscosity.
BEAVERTAIL BAFFLE
-
The flange-mounted Beavertail baffle with temperature sensing system offers high performance mixing even at low liquid levels
The high performance Beavertail Baffle
CHARACTERISTICS
The Beavertail baffle is flange-mounted allowing easy, no-entry installation and removal. It can be installed through one of the standard nozzles on a De Dietrich reactor as well as any other glass-lined reactor. No special holder is required for the baffle. Our DR type temperature sensor comes standard.
The flange-mounted design reduces the possibility of leaks through gaskets, since only one gasket is required for a seal.
Sized to fit vessels from 370 liters to 110,000 liters (popular sizes from 370 liters to 20,000 liters are normally available from stock for quick shipment).
Headroom approximately equal to the length of the baffle is required.
ADVANTAGES- No need to enter vessel or remove contents to install or change-out the baffle
- Chance of leaks substantially reduced with only one gasket required for a seal
- Sensor change out/maintenance while vessel is in service
- High performance mixing even at low liquid levels

DIP PIPE BAFFLE
-
The innovative combination dip pipe/baffle provides four functions in one piece of equipment.
Baffling, product introduction, sampling and temperature measurement can all be performed from a single nozzle, freeing up additional nozzles for other functions.
Installation requires a DN200 (8”) nozzle or larger. A bellow or expansion joint must be used in conjunction with the baffle/dip pipe to compensate the thermal expansion of the inner pipe.
ADVANTAGES- Flange-mounted for easy and inexpensive installation without having to enter the vessel and cause unwanted delays in production.
- Completely lined with DD 3009 glass, the unit has optimum corrosion resistance and meets cGMP requirements for cleaning.
- The temperature sensor can be removed or replaced while the vessel is still in service.

MULTIPROBE® SAMPLING SYSTEM
-
The exclusive design of the MultiProbe system allows a perfectly representative sample to be "captured" in the very heart of the reaction process
The easiest way to take a representative sample without opening the manway!
CHARACTERISTICS
Once installed, the MultiProbe is immediately operational without need of further adjustment. Its design facilitates its installation on small reaction units. Its compact over-all dimensions make it possible to be integrated in a congested peripheral environment.
The MultiProbe is able to fully clean the entire sampling circuit with the use of an appropriate solvent.
The ability to take a truly representative sample with no need to depressurize the vessel or lose the insert atmosphere makes this product ideal for chemical and pharmaceutical processes where contamination is usually an issue during sampling.
ADVANTAGES- Representative Samples
- Minimized Downtime
- Health and Safety Benefits
- Process Control
- Multipurpose Functionality
- No Additional Support
Distillation
- Separation of chemical components is often achieved by using distillation or evaporation. De Dietrich Process Systems has designed such equipment for many years within the QVF brand of our integrated business while the De Dietrich glass lining brand has designed and built many distillation columns over the years.

EVAPORATORS
- Evaporation is a common activity and we have a range of thin film evaporators for heat sensitive materials as well as the more basic heated vessels with condensers and receivers.
PROCESS SOLUTIONS FOR THERMAL SEPARATION
-
Columns and internals for aggressive media
HIGHLIGHTS- Corrosion-resistant materials for corrosive substances
- Vacuum and pressure-resistant for a wide variety of process conditions
- Temperature ranges up to +230°C
- Diffusion-proof for sustained corrosion resistance
- Smooth surfaces that stay clean, non-stick effect
- Abrasion-resistant for a long service life
- Compact design to keep investment costs down
- Reliable process design for further investment cost reduction
- Solutions optimised for efficient processes
- From initial design to component manufacturing to commissioning – from a one-stop shop
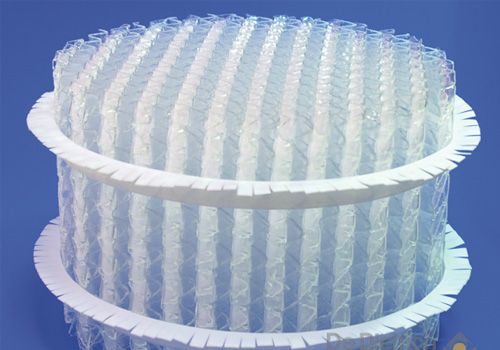
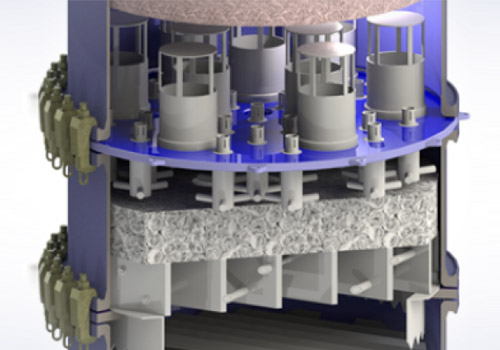
STRUCTURED PACKING DURAPACK®
-
- made of borosilicate glass 3.3
- highly corrosion resistant
- high separation efficiency-
- low pressure dropThe efficient solution for highly corrosive thermal separation processes
CONCEPTThermal separation processes are often used in treating and cleaning chemicals. Very common are processes that involve two liquids or a liquid and a gas. The processes used mainly involve extraction, absorption, desorption or stripping, distillation or rectification. These separation processes require mass transfer between two phases; in the above rectification example, the mass transfer between the liquid and gas phase. Keeping the equipment used in this process as compact as possible requires creating as much mass transfer surface in as small a volume as possible. DURAPACK® ensures high flow rates with low pressure drop and at the same time huge mass transfer areas for an efficient separation.
In order to intensify the mass transfer in absorption, desorption, distillation and extraction columns structured packing DURAPACK® from borosilicate glass 3.3 is the best choice for corrosive media. Applications are among other are therefore corrosive chemicals such as sulfuric acid, nitric acid, other mineral acids such as HCl but also halides such as chlorine, bromine and iodine: short wherever metal material but also plastic fail.
DURAPACK® patented structured packing consists of alternately allocated corrugated plates made of borosilicate glass 3.3 with channels at an angle of 45°. In order to increase the turbulence the corrugations are notched. The packing is only made from borosilicate glass 3.3, i.e. the plates are sintered together without any gluing material so that only borosilicate glass 3.3 is in contact with the media to be processed.
The easy-to-clean smooth and inert surface is extremely convenient.
EXTRACTION
-
The dissolving process of a chemical component with a liquid out of a second non-gaseous phase is called extraction. The dissolving process of a chemical component with a liquid out of a second non-gaseous phase is called extraction. Depending on the kind of the second phase this process is named either solid/liquid extraction or liquid/liquid extraction.
Liquid/Liquid Extraction Solutions:
- Batch operated Mixing/Settling Units
- Continuously operated Mixer-Settlers
- Continuously operated Extraction columns
Solid/Liquid Extraction Solutions:
- Soxhlet Extraction Units
- Trickle Bed Extraction Unit
- Over Flow Extraction Units

SOLID/LIQUID EXTRACTION
-
Solid/Liquid extraction process is a very common process in the pharmaceutical, cosmetic and food industry to obtain natural ingredients as e.g. flavors and fragrances from natural raw material.
The extraction can be carried out with cold or hot solvents.
The solid raw material is packed in a container with a retainer on the bottom called extractor B2 and extracted batch wise.
The solvent is guided through the extractor in different ways
- Continuous trickle bed extraction
- Continuous overflow extraction
- Soxhlet extraction (periodically filled and drained container)
Using solvents with a lower density than the raw material avoids floating of the raw material and eases the process.
Very often the solvent is evaporated in B1 from the extract directly after leaving the extractor, then condensed in W2 and guided back into the extractor B2.
LIQUID/LIQUID EXTRACTION - Batch mode - Mixers/Reactors
-
Whereas distillation takes advantage of different volatilities means different distributions of a product in the liquid and the gas phase the liquid/liquid extraction is based on different solubilities means different distributions of a product in 2 co-existing liquid phases.
For the extraction of a product (white dots) out of the so called feed liquor (blue liquid with white dots) a suitable solvent (yellow liquid) has therefore to be found. The first step of an extraction process is mixing for an intensive contact of both liquid phases to enable the mass transfer of the product (white dots) from the (blue) feed liquor into the (yellow) solvent. The second step is the phase separation or settling of the 2 liquid phases. After the extraction of the product the feed liquor is called raffinate (blue liquid with less white dots) whereas the solvent containing the product is called extract (yellow liquid with white dots). For the recovery of the product the solvent has to be separated in a subsequent third step from the product which is mostly done by distillation
Batch mode - Mixers/Reactors.
Using a mixer in batch mode offers a huge flexibility to optimize the mass transfer by varying ratio of the liquid phases, the type of stirrer, stirrer speed and mixing time. The settling period can also be easily influenced by varying the time. Such a batch operation is labor sensitive and requires sufficiently big equipment.
What can be done in the laboratory scale with a separation funnel can be realized in a bigger scale with larger quantities in a more defined way by using tempered mixers having basically the same functionality as batch reactors for reactions in the liquid phase. This is especially advantageous for the extraction of reaction mixture as it can be carried out in the same equipment as the reaction. Mixing can be adjusted by the stirrer shape, speed and mixing time. The phase separation is a question of time and can be observed advantageously in glass reactors. For the phase separation it is necessary to determine the location of the interphase. In the reactor this can be measured by systems based on a floater, a radar signal or the conductivity depending on the system to be examined. Outside the reactor it is also possible to detect the interphase while draining the lower means heavier phase through the bottom outlet by visual inspection through a glass pipe or by the sudden change of media property.
VACUUM DRYING SOLUTIONS
- Quick cycle times, excellent product homogeneity and low residual moisture: this characterizes De Dietrich Process Systems agitated vacuum drying solutions.

PAN DRYER
-
The Guedu® Pan Dryer is adapted for dynamic vacuum drying of delicate, heatsensitive product, requiring low residual moisture. The agitated drying allows a good mixing and a thermal homogeneity which ensures even drying.
The Powerful high performer
FLEXIBLE :
A robust, proven design, the Guedu dryer is adapted to all conditions of use.
EFFICIENT DRYING :
The design of the agitation is specially developed to facilitate the drying, with a high product renewal rate on the heated surfaces and to ensure efficient heat transfer.
COMPACT :
The design has an excellent volume to footprint ratio, simplifying installation in your existing plant.

CONICAL DRYER
-
The De Dietrich conical dryer is adapted for dynamic vacuum drying of delicate, heat sensitive product, requiring low residual moisture. The agitated drying allows a good mixing and a thermal homogeneity which ensures even drying
FULL DRAINAGE :
Drainage is facilitated by the vertical design.
LOW MINIMUM AGITATED VOLUME :
The minimum agitated volume is very low compared to the capacity of the equipment, which allows to use the same equipment for very different batch volumes.
FLEXIBILITY AND CLEANABILITY :
Thanks to the full discharge, the risk of cross-contamination is limited which give production flexibility. Additionally, retention zone are limited.
Thanks to the hydroformed jacket technology, the heat transfer is optimised and heat transfer performance is improved by up to +30% compare to previous jacket technologies.
Flexibility & cleanability : Thanks to the full discharge, the risk of cross-contamination is limited which give production flexibility. Additionally, retention zone are limited. Thanks to the hydroformed jacket technology, the heat transfer is optimised and heat transfer performance is improved by up to +30% compare to previous jacket technologies.
DRYING PERFORMANCE :
The agitator is specially designed to facilitate the drying, with high product renewal rate on the heated surfaces to ensure efficient heating exchange.

SPHERICAL DRYERS
-
The Spherical Dryer is a highly engineered and precision fabricated vacuum dryer designed to utilize heat input, effective mixing and vacuum for product drying.
Dedicated or multi-purpose applications : the best in product quality, total discharge, cGMP production, cleanability.
The trend setting spherical dryer range with top or bottom drive is the latest development of the proven Rosenmund® dryers that achieve the highest performance standards. A versatile solution for drying, mixing and granulating.
IMPORTANT ADVANTAGES AT A GLANCE- Drying, mixing and granulating.
- Fast and easy cleaning (CIP/WIP and SIP).
- Total product discharge.
- Short drying time.
- Reproducible drying result.
- Heatable agitator.
- Vertical chopper/lumpbreaker.
- Easy to inspect.
- Assembly in ceiling.
- Containment of explosion pressure surges.

UNIVERSAL DRYERS
-
The innovation: agitator and chopper in one system.
Unequalled application spectrum and extremely short drying times, with the highest possible fill volume.
MAXIMUM FLEXIBLITY AND EFFICIENCY
Maximum heat transfer input to the product is achieved by heating the total vessel area and the agitator. A further advantage is the formation of an especially fine product powder in the dryer, which accelerates the heat transfer and thus the drying. The particle size of the product can be decrease further by the addition of a fixed stator to the agitator system.
ADVANTAGES- Chopper integrated in the agitator arm.
- Reliable lumpbreaking.
- Small particle / grain size.
- Wet and dry milling.
- Drying independent of the initial product consistency.
- Small agitator to wall clearance.
- Hinged door for easy inspection.
- Full-surface heating of the agitator and vessel.
- High torque means maximum filling volume.
- Complete encasing of the drive system.
- Single-nozzle device on the agitator for applying
- Nitrogen or liquids for granulation.
- Pilot scale trials can be conducted in the Rosenmund technical centre.

DOUBLE CONICAL DRYERS
-
The double conical dryer type SR (glass lined or in stainless steel) is a device that was designed for drying easily flowing products.
The rotation of the double cone allows the product to be mixed without an internal agitator.
FIELD PROVEN AND RELIABLE: THE DRYER PRINCIPLE.
A proven concept
The design of the double conical dryer combines the drying and mixing function in one simple and stable device. The operation and maintenance costs are very low. The drying times are reduced to an acceptable level. As a result, this concept provides a simple solution for many drying requirements.
IMPORTANT ADVANTAGES AT A GLANCE- Functional design.
- Simple operation.
- Problem-free cleaning.
- Low operating costs.
- Wide range of options.
POWDER HANDLING
- Powder handling and containment are critical aspects of pharmaceutical and chemical processes. In addition to conveying powder in an efficient way, there is also the need to keep the product free from contamination as well as keep the operator free from exposure to the product, especially when hazardous media is involved.

POWDER PUMP
-
The solution for conveying/charging powder from one piece of equipment to another safely and efficiently.
The simple and reliable solution for powder transfer
DESCRIPTION
The Powder Pump safely and efficiently moves powder from one piece of equipment to another using a process known as dense phase flow.
Its design, construction and simple operation enable it to fit easily into your environment.
The technology makes the Powder Pump an ideal solution for controlled material addition. Suitable for the transfer of raw, intermediate or finished products, it is a versatile work horse for the chemical and pharmaceutical industries, as well as other industries with various solid handling needs.
CONTAINMENT- No dust formation
- Powder can be transferred into a reactor without opening a manway, retaining the vessel's inert atmosphere
- Powder can be charged into a reactor under pressure or vacuum or with solvent already present in the vessel
SAFETY- Operator no longer exposed to a reactive environment
- Operator exposure to the powder is greatly reduced
- No introduction of oxygen into the enclosure being loaded
- Creation of electrostatic charges is strongly reduced

POWDER BLENDER
-
In a cylindrical vessel with a flat base, an agitator with a unique profile provides a full, constant and efficient mixing of the entire product.
The Guedu® mixing agitator creates a vortex motion which creates 3 separate mixing effects on the product: division, separation by thin layer and dispersion effects. Charging is done from the top. Discharge is done either through a manual or automatic side discharge door or valve.
The Guedu® mixing agitator creates a vortex motion which creates 3 separate mixing effects on the product: division, separation by thin layer and dispersion effects. Charging is done from the top. Discharge is done either through a manual or automatic side discharge door or valve.
The base of the vessel and the underside of the agitator are completely flat. The unit is built with minimal clearance between the vessel and agitator; to allow full discharge of the product with minimal carry-over between batches.
The construction avoids all dead spaces :- Agitator profile adapted to suit the product type (solid, liquid, paste)
- Discharge door flush to the inside vessel wall
- Spray ring for product wetting or full wash-in-place
Depending on the device size, the product and the desired efficiency, one or more high-speed lump breakers are installed. Lump breakers can be either side-mounted (through the vessel wall) or top-mounted (retractable device fitted on top of the vessel).
HEAT TRANSFER
-
Heat transfer is essential to the reaction process, whether it is used to heat up batch reactors, control exothermic reactions, condense vapors and gases or cool down final products.
DESCRIPTION
Combining our process engineering knowledge with highly corrosion-resistant materials allows us to provide many high-performance heat exchangers and condensers for difficult applications.
By selecting the appropriate materials for your chemical application and sizing the heat exchanger using computer simulations for your specific process flows and requirements, we can provide many different heat exchanger designs.
We also provide skid-mounted heating and cooling systems for reactors, dryers and filter-dryers that utilize either engineered heat transfer fluids or conventional steam/water/brine circuits for temperature control of equipment. 
EC TYPE
-
From 2 ==> 14 m2
The EC type exchanger is mainly used as a condenser.
In conjunction with our reactors, it is possible to make up distillation ranges of any composition
Its design is plain and permits to achieve great exchange areas with a small bulk. This exchanger can also be designed to serve as a heater or and evaporator.

ED TYPE: JACKETED PIPE HEAT EXCHANGER
- DN 25 ==> DN 250

QVF® SUPRA SHELL AND TUBE HEAT EXCHANGERS
-
- Medium side corrosion resistant - service side up to 6barg -
- Both sides corrosion resistant up to 3barg -
- Suitable for pharmaceutical production -
The large heat transfer surfaces and high heat transfer coefficients of shell & tube heat exchangers permit the transfer of larger amounts of heat than is possible with coil type heat exchangers
HIGHLIGHTS- Both sides resp. tubes and shell resistant to corrosion
- Pressure-proof (medium or service side) up to +6 barg
- Suitable for pharmaceutical products
The QVF SUPRA shell and tube heat exchangers are available with
- nominal diameters of DN80 to DN300
- heat exchange surfaces of 0.3m² to 27m²
- tubes made of borosilicate glass 3.3 or SiC
- shells made of borosilicate glass 3.3, glass-lined steel or stainless steel
- headers made of borosilicate glass 3.3, glass-lined steel or stainless steel

CONDENSATION AND SEPARATION UNIT THERMIPACK®
-
The condensation and separation unit Thermipack® offers a combination of different process steps: Condensation, first and second steps, Separation, Storage of solvents in one single device.
Thanks to the modular design, the decanter can be used only if required, or it can be employed to complement another existing unit. Other functions, such as additional VOC (Volatile Organic Compounds) recovery, can be easily integrated into the Thermipack® unit. The materials (enamel, stainless steel, alloy) are chosen according to the characteristics of the products to be treated.
EASY CLEANING
Complete internal cleaning of all components, a low retention of liquids as well as smooth inside and outside surfaces guarantee complete and efficient cleaning according to FDA requirements. Additionally installed spray nozzles guarantee reliable cleaning even without disassembly.
COMPACTNESS
The unmatched compactness of Thermipack® leads to a space requirement 4 times smaller than the one of a conventional design. If there is only little space available, Thermipack® is a must.
LESS PIPEWORK
The connection pipes are reduced by up to 80%.
LOWER INVESTMENT COSTS
Less plot space, significant reduction and simplification of piping with less support structure, reduced handling and installation minimise project costs.
REDUCED VOC
The efficiency of the two stage plate condensers reduce the quantity of uncondensed solvent.
SOLVENT HANDLING
Since larger quantities of solvent can be stored, handling efforts are reduced and the full batch can be analyzed and pre-treated in situ.
THE RANGE
3 sizes of condensing units covering a range of reactors up to 16m2.

OPTIMIX® - HE (HEAT EXCHANGE)
-
Through the evolution of the OptiMix design, De Dietrich Process Sytems has extended the range of the OptiMix reactors to provide improved heat transfer and reduce processing times.
Increase your heat exchange surface with the new generation of OptiMix - HE reactors
CHARACTERISTICS:- A complete range from 100 l up to 20,000 l in half-coils with thermal fluid
- Geometry according to DIN 28136
- Inside: -25/+200°C, -1/+6 bar / Outside: -25/+200°C, -1/+6 bar
- Heated / cooled baffles
- DD3009 Enamel
ADVANTAGES- Heat exchange area increased up to 25%
- Reduced reaction time
- Clearance of all the nozzles
- Improved cleaning facilities:
- No dead zone
- Less vortex means reduced splashing on wall and upper head

CORE-THERM
-
- High pressure heat exchanger -
- Pressure resistant on product and service side -
- Up to +10barg between -40 to + 200°C -
- Corrosion resistant on product and service side -
- SiC-Tubes -
The fully corrosion resistant heat exchanger for the really tough conditions!
DESCRIPTION AND FEATURES:- Single tube seal with double clamp rings
- Diffusion-resistant tube plate
- DN 100-DN 300: -1/+10 bar, -40/+200°C
- 0,4-20 m² heat transfer area
Heat exchangers made of inert, non-metallic materials are a requirement in the chemical and pharmaceutical industries where it is essential to avoid any interaction between the materials of construction and the substances being processed. In addition to chemical resistance there is a prime requirement for resistance against abrasion and easy of cleaning of the equipment.
It is usually not possible to fuse-join or weld non-metallic materials which meet these requirements, so that the tightness of the heat exchanger depends on the quality of the sealing between the inner tubes and the tube plate.
Considering the different thermal expansion coefficients and the possible temperature differences in the equipment these seals must be capable of taking up any linear expansion that occurs.
STORAGE
-
De Dietrich Process Systems tanks and receivers are cost effective solutions for chemical storage.
Lined with DD 3009 glass, they are ideal for the storage and containment of corrosive chemicals or high purity pharmaceuticals.
STORAGE TANKS:
The glass-lined steel storage tanks can be designed for a working pressure of -1 / +6 bar and are equipped with manholes and nozzles according to your requests.
Three series of glass-lined steel tanks are offered in various sizes to fill all plant storage needs – clamped-top CR series vertical tanks and closed-welded VT (vertical) and HT (horizontal) series.
RECEIVERS:
In standard execution our receivers are designed for an inner pressure of -1/+6 bar and 6 bar in the jacket if needed. 
TANKS
-
The glass-lined steel storage tanks can be designed for a working pressure of -1 / +6 bar and are equipped with manholes and nozzles according to your requests.
Safe and reliable chemical storage
DESCRIPTION
Two series of glass-lined steel tanks are offered in various sizes to fill all plant storage needs. (Vertical and horizontal tanks).
ADVANTAGES- DD3009 enamel
- High resistance to corrosion
- Reliability

RECEIVERS
-
Our receivers are designed for an inner pressure of 6 bar/-vacuum and, if needed, 6 bar in the jacket.
Equipment to complete your installations
TWO RANGES OF RECEIVERS:- A range from 50 to 800 liters with cover and clamps
- A closed range from 1200 to 6000 liters with a manhole
ADVANTAGES:- DD3009 enamel
- High resistance to corrosion
- Reliability
CONTAINMENT
Containment
-
DESCRIPTION
The handling of highly potent materials requires an additional level of operator protection compared to traditional manufacturing. While this applies to all areas of the plant, De Dietrich process equipment focuses specially on:- Reactor charging
- Filter dryer offloading
- Product packaging
The primary form of protection is via a physical barrier between the operator and the product, while a pressure differential or managed airflow creates a secondary barrier.
A ONE STOP SHOP
A containment system can be integrated with De Dietrich process equipment, and works seamlessly by combining interfaces, automation systems, safety interlocks and other peripheral components.
Isolators have the following advantages
- Creation of a microenvironment limiting impact on the cleanroom.
- Reduction of the need for PPE (Personal Protective Equipment).
- Limited total life cycle cost.
PIPING & VALVES
-
e Dietrich Process Systems has a complete line of valves, pipes and fittings constructed out of extremely corrosion resistant materials.
Used in diverse industries for multi-purpose applications, our equipment is resistant to almost all chemical attacks, assuring product purity and providing high durability. 
GLASS-LINED PIPING
-
Covered with DD3009 enamel, our glass-lined pipes and fittings are made with the
same care used on all our equipment, giving the optimum properties of mechanical
and corrosion resistance.
The safest and most efficient solution for your fluid transfer.
The transportation of fluids is essential in a process, which is why it's important to have the same corrosion resistance in your pipes and fittings as your other equipment.
Whether for standard or special parts, our glass-lined piping range benefits from the same enamel quality as used for our reactors. This allows us to meet the demands of the chemical and pharmaceutical industries.
CHARACTERISTICS- Dimensions and tolerances according to DIN2873 or ASME150
- Enamel DD3009 / Lining thickness according to ISO 28721-4 (btw 0.8mm and 2mm)
- Pressure: -1 up to 10 bar
- Temperature: -25°C up to +260°C
- Painting as per customer specification
- Earthing solution on all our products
- CE marking for the whole range of piping
ADVANTAGES- Designed to resist high stresses and avoid enamel breakage
- Same enamel quality as used for our reactors
- High resistance to corrosive media, acids, abrasion, high temperature
- Easy to clean
- Non-permeable lining
- Not subject to liner collapse due to full vacuum at high températures

CLEANVALVE
-
De Dietrich Process Systems carries a range of valves that effectively control the flow of liquids, gases and slurries in reactors, tanks and other process equipment. Cleanvalve is ideal to use in any process where batch to batch cleanability is important.
Cleanvalve, the easiest to clean and self draining bottom outlet valve.
Cleanvalve is a bottom flush valve designed for use on glass-lined, Stainless Steel or Nickel Alloy reactors / Tanks. This easy-to-operate, easy-to-clean, self-draining valve allows for a variety of functions without the need to interrupt the process and without dismantling the valve.
Advantages
The glass-lined steel design of the Clean Valve ensures no metal contamination of the process fluid and enables use across a wide temperature and pressure range. Cleanvalve has been designed with the most rigorous cleaning operations in mind.- Patented flat seat to avoid collection or build-up of materials in the annular area between the valve seat and nozzle wall.
- Additional port with a 5º downward sloping angle has been built into the valve to facilitate cleaning of the internal components and body.
- Retractable spray nozzle system
- Cleanvalve is able to accommodate temperature measurement sensors, measurement electrodes at the lowest point in the vessel for a more accurate and reliable temperature measurement.
- Lateral dismantling of the temperature probe
- All DDPS glass-lined valves are made of cast steel coated with our standard 3009 Glass enamel identical to the reactors on which they are installed. Wetted parts feature the same characteristics of all other glass-lined equipment including resistance to corrosion, thermal and mechanical shock. All other parts in contact with the process are made of fluoropolymer (PTFE, PFA).
- Cleanvalve can be fitted with lots of accessories (limit switch, solenoid valve…)
- For standard nozzle or block flange
- The same spare parts for all materials
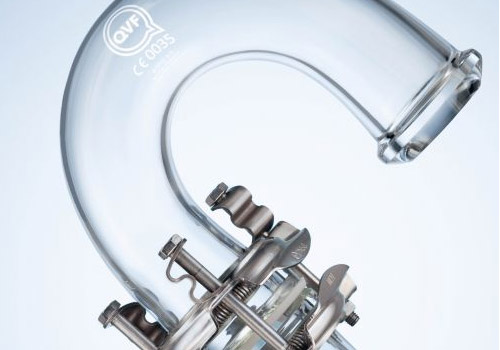
QVF® SUPRA LINE - THE COMPONENT SYSTEM
-
QVF SUPRA-Line can not only connect the former glass systems to the QVF SUPRA-Line but also to interconnect the different former glass systems to each other.
THE SUPERIOR COMPONENT SYSTEM:
ENAMEL & BOROSILICATE GLASS
ENAMEL PROPERTIES
-
The use of glass-lined steel is mandatory when service conditions of the process are particularly difficult and demanding.
RESEARCH AND DEVELOPMENT
De Dietrich Process Systems has always invested in research and development of new glass formulas with greater capabilities. The result of our ongoing research enabled us to offer DD 3009 glass and ConductiGlass. 
ENAMEL DD 3009
-
The DD 3009 glass is a material of construction which meets the demanding needs for chemical and pharmaceutical companies where service conditions of the process are particularly difficult.
One glass with optimum quality
WHAT ARE THE PROPERTIES OF DD3009 GLASS?
DD3009 glass offers excellent resistance to corrosion, abrasion, mechanical and thermal shocks, making it suitable for highly corrosive processes. The formulation of this multipurpose glass is adapted to cGMP requirements and its anti-adhesive properties are ideal for cleaning, cleanliness, and sterilization.
Furthermore, the glass surface is impervious to catalytic effects and contamination.
DD3009 GLASS ADVANTAGES- Excellent resistance to corrosion
- Mechanical resistance to shocks and abrasion
- Smooth, non-stick properties
- Non-catalytic inertness
- Multipurpose material for versatility
- Meets cGMP requirements for cleaning, cleanliness and sterilization
- Suitable for high pressure and full vacuum at elevated temperatures

CONDUCTIGLASS
-
ConductiGlass® enamel prevents any risk of electrostatic damage on glass-lined equipment
The solution against electrostatic damage
In a glass-lined reactor, electrical insulating properties of the lining prohibit charges to flow to the ground and enhance their accumulation to very high voltages that become dangerous for the glass lining. This problem which was one of the last remaining in glass lining technology is solved by the use of the glass lining called ConductiGlass.
The principle of the ConductiGlass solution consists in increasing the conductivity of the glass lining to a value high enough to allow the discharge to the ground the electrostatic charges created, prohibiting then their accumulation at the glass surface up to dangerous voltages.
This is achieved by adding a small quantity of very thin Platinum fibres, homogeneously dispersed in the whole thickness of the glass lining, which remains made of DD3009 standard glass.
These fibres are not joined, but the glass thicknesses which are in between them are extremely small and can be compared to a network of micro-condensers discharging in line to let the electrostatic charges flow gently to the ground without any damage to the glass lining.
The first ConductiGlass equipment have been installed in 1998, and more than 100 reactors are working actually, replacing vessels previously damaged by electrostatic discharges, in activities as different as production of medicines, pesticides, resins, electronic components, using solvents like toluene, chloroform, ether, n-heptane, benzene, …
ConductiGlass advantages- Delete any risk of electrostatic damages
- Easy to implement on any kind of glass-lined equipment
- Continuous intrinsic property of the whole thickness of the glass-lining during all the life-time of the equipment
- Dielectric controls and Email Test monitoring remain possible
LIQUID-SOLID SEPARATION
-
From lab to pilot and full production scale,
De Dietrich Process Systems filtration solutions are highly versatile and adaptable to many chemical processes (pharmaceuticals, chemicals, natural extraction, precious metals, dyes...)
DESCRIPTION
Our team of process experts work with you to define your requirements, evaluate feasibility, proceed through small scale trials and scale-up calculations.
From the smallest company to the largest system, from the simplest machine to the most complicated, we are here to help you define your needs.
Filter-dryers can be used for the following batch process steps:- Filtration (formation of the cake)
- Cake smoothing
- Cake washing (piston or reslurry washing)
- Vacuum drying
- Cooling
- Automated discharge

STATIC NUTSCHE FILTERS
-
Static liquid-solid separation - that is the purpose of Nutsche filters. Fitted with a filtration media at the bottom and suitable for vacuum of pressure applications, Nutsche filters are a larger scale version of the well known lab Büchner filter
Whether manufactured in QVF glass, De Dietrich glass-lined steel, stainless steel or hastelloy, Nutsched filters all follow the same principle. A filter media is placed at the bottom of the vessel, allowing a slurry to be loaded and the solids (usually crystals) to be separated from their mother liquor.

PILOT SCALE FILTER-DRYER : ROLAB
-
Easy to operate and highly portable agitated nutsche filter, designed for the filtration and drying of small pharmaceutical and chemical batches.
It is a manual solution for both laboratory and production environments that provides all the process functionality of filter-dryers
The simple and effective pilot-filter/dryer: RoLab
RoLab is a standardised unit designed for pilot scale, multi-product environments. Rolab is mobile, easy to install and operate, and available with filtration areas between 0.03 and 0.4 m². The standardised design allows significant cost saving compared to custom built units.
RoLab allows users to carry out all steps of a professional modern filtration and drying procedure under cGMP and FDA guidelines. The installation enables fast and frequent product changes and can be flexible due to its mobility. These features result in a high cost-effectiveness of the testing and manufacturing processes as well as the used capital goods.
RoLab impresses with its simplicity and focus on the essential. The machine has no hydraulic system and the extent of electronic function support is reduced to a minimum. The installation of RoLab is straightforward and fast. The filter/dryer only needs to be connected by the user with the supply lines:
• Compressed air
• Electricity
• Heating/Cooling
Subsequently, the process connections (suspension, nitrogen,
etc.) have to be attached and the RoLab is ready for operation.

GLASS NUTSCHE FILTER DN600
-
HIGHLIGHTS
- Superior technical solution
- From DN300 to DN600
- No metal in contact with the product
- Two separate movable parts
- Comfortable handling
- Easy cleaning
- Quick product collection
- Simple substitution of the filter cloth
- Connections for ventilation, nitrogen etc.
- Excellent corrosion-resistance
- Smooth, pore-free surface
- Transparency
- Catalytic inertness
- No effect on odour and taste
- Physiological acceptability
CONCEPT
The brand new QVF®-Nutsche Filter for vacuum filtration ideally meets the special demands of R&D as well as small scale production in the Fine Chemical and Pharmaceutical industry.
The two main parts of the QVF®-Nutsche Filter system- mobile filter plate
- glass cover with height adjustable blade to smoothen the filter cake
are mounted on wheels, so that the unit can be comfortably moved to different locations

LABORATORY POCKET FILTER
-
The Pocket Filter performs fast, safe demonstrations and tests of filtration, washing and drying. Stuiable for table top installation
The Pocket Filter is ideal for chemical and pharmaceutical companies looking to make smarter, more cost effective scale-up choices.
POCKET FILTER ADVANTAGES
Purposely designed for bench-top filtration testing, it offers a number of advantages:- Mobility – Small and easy to transport, you always have the right tool in the right place.
- Laboratory Tests - The Pocket Filter kit contains all you need to perform laboratory tests simply and safely.
- Filtration tests – perform pressure and vacuum filtration testing. The tests yield a filtration rate and cake depth as well as the optimizing of filtration pressure, filter media and filtration time.
- Washing tests – demonstrate the relative efficiencies of displacement versus reslurry washing.
- Drying tests – measure the feasibility of vacuum and pressure gas drying while circulating hot fluid throughout the jacket.
- New Application Data - The Pocket Filter gives you daily support in the laboratory and provides valuable data for any new application before it is tried at full scale.
- Full-Scale Options - The Pocket Filter allows you to examine the effect on your filtration rate (and therefore your batch time) of varying pressures and different types of filter media, such as cloth versus sintered metal, or micron rating. Determining the optimum solution on the bench-top is far easier than in the midst of a full-scale production campaign!
- Process Optimization - Test data are frequently the basis for process improvements.
- Cost Effective - With a minimum of investment you obtain data which lead quickly and safely to the best solution.

AGITATED NUTSCHE FILTER AND FILTER-DRYER
-
The De Dietrich Process Systems Nutsche Filter and Filter-Dryer technology is particularly suited to meet the stringent requirements of the pharmaceutical and fine chemicals industries for solids washing and separation, even in the most challenging process conditions.
Tailored to your needs. From the smallest to the largest, from the most simple to the extremely complex filter-dryer.
VERSATILE FILTRATION AND DRYING
The filter/dryer performs a multitude of tasks including filtration, displacement or reslurry washing, vacuum or convection drying. It can discharge wet cake, slurry, liquid, or dried cake to less than 0.1% moisture.
Filter-Dryers have numerous advantages :- Limited overall dimensions
- Reduced investment cost
- Improved product quality
- Reduced cross contamination risk
- Closed system : no operator contact, no product transfer from one machine to another.
FILTER-DRYER ADVANTAGES
Our filters and filter/dryers have many features that make them the recognized industry leader including :
- Universal Filter Media - The filter plate design allows the use of all types of filter media: cloth, single later metal screen, or multi-layer sintered metal.
- Side Discharge Valve - Metal-to-metal seals with minimal dead space, eliminating the cleanability issues that can arise with the use of o-rings. The valve provides a pressure/vacuum-tight seal.
- Heated Filter Plate - The fully welded design provides heat energy directly into the cake from the underside, allowing for heat transfer through all of the filter contact surfaces, for more efficient drying.
- Gas-knife system ; small nozzles located underneath the S-blade agitator blow the heel of the product towards the discharge valve.
